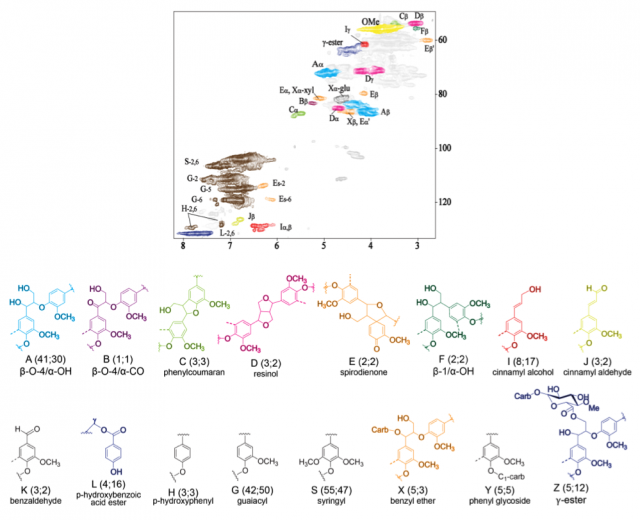
The intact linkage structures and subunits within the lignin polymer can now be resolved by high resolution 2D HSQC NMR based on 1H-13C correlations (Ammalahti et al., 1998; Ralph et al., 1999, 2006, 2008; Ralph and Lu, 2004; Capanema et al., 2004, 2005; Hu et al., 2006; Balakshin et al., 2007, 2008; Zhang and Gellerstedt, 2007). Approximately 85% of the lignin interunit linkages have been identified (see Figure; Capanema et al., 2004, 2005). Absolute quantitation of composition and linkages can be obtained from a 1D 13C and a 2D HSQC spectrum (Capanema et al., 2004, 2005; Ralph et al., 2006; Zhang and Gellerstedt, 2007). β-ethers (β-O-4) account for 40 to 65% of the total linkages in wood lignins (Adler, 1977; Ralph et al., 2004, 2008; Balakshin et al., 2007; Capanema et al., 2005; Zhang and Gellerstedt, 2007). Other linkages include α-ethers (α-O-4) and C-C types, such as β-5, β-β, β-1 and 5-5. Atypical monomers in lignin have also been detected by NMR. Most if not all linkage types have been corroborated as PO-catalyzed oxidative coupling products (Rasmussen et al., 1995; Takahama, 1995; Kim et al., 2000, 2003; Barcelo and Pomar, 2001; Ralph et al., 2004a, 2004b, 2008; Holmgren et al., 2006; Bunzel et al., 2008).
Figure. HSQC NMR spectrum of “Cellulolytic Enzyme Lignin” (CEL, Chang et al. 1975. Holzforschung, 29:153-159.) isolated from the stem wood of wildtype P. trichocarpa. Contour colors correspond with the lignin inter-unit linkages shown. Linkages were quantified by 13C and HSQC and compared with those from 4CL down-regulated P. trichocarpa. Values (wildtype; transgenic) are quantity/100 monomeric lignin units. Y and Z were quantified based on the NMR spectra of crude milled-wood-lignins from the wildtype and transgenic.
References
Adler, E. 1977. Lignin chemistry – past, present and future. Wood Sci. Technol. 11:169-218.
Ammalahti, E., et al. 1998. J. Agric. Food Chem. 46:5113-5117.
Balakshin, M. Y., Capanema, E. A. and and Chang, H. 2008. Recent advances in isolation and analysis of lignins and lignin-carbohydrate complexes. In: Characterization of Lignocellulosics. T. Hu (ed.). Blackwell Publishing, Oxford, UK.
Balakshin, M. Yu., Capanema, E. A., Chang, H.-m. 2007. Holzforschung. 61:1-7.
Bunzel, M., et al. 2008. J. Agric. Food Chem. 56:10368-10375.
Capanema, E. A., et al. 2004. J. Agric. Food Chem. 52:1850-1860.
Capanema, E. A., et al. 2005. J. Agric. Food Chem. 53:9639-9649.
Chang, H.M., et al. 1975. Holzforschuing 29:153-159.
Holmgren, A., et al. 2006. Org. Biomol. Chem. 4:3456-3461.
Hu, Z., et al. 2006. Holzforschung. 60:389-397.
Kim, H., et al. 2000. Org. Lett. 2:2197-2200.
Kim, H., et al. 2003. Org. Biomol. Chem. 1:268-281.
Ralph, J. and Lu, F. 2004. Org. Biomol. Chem. 2:2714-2715.
Ralph, J. et al. 1999. Solution-state NMR of lignins. In: Argyropoulos, D. S. (ed.) Advances in Lignocellulosics Characterization. D. S. Argyropoulos. In: Advances in Lignocellulosics Characterization.Tappi Press, Atlanta, GA.
Ralph, J., et al. 2004. Phytochem. Rev. 3:29-60.
Ralph, J., et al. 2004. Phytochemistry Rev. 3:79-96.
Ralph, J., et al. 2006. J. Biol. Chem. 281:8843-8853.
Ralph, J., et al. 2008. Lignification: Are lignins biosynthesized via simple combinatorial chemistry or via proteinaceous control and template replication? In F. Daayf, A. El Hadrami, L. Adam and G. M. and Ballance (eds.), Recent Advances in Polyphenol Research. Wiley-Blackwell Publishing, Oxford, UK.
Rasmussen, C. B., Dunford, H. B. and Welinder, K. G. 1995. Biochemistry. 34:4022-4029.
Ros Barceló, A. and Pomar, F. 2001. Phytochem. 57:1105-1113.
Takahama, U. 1995. Physiol Plantarum. 93:61-68.
Zhang L. and Gellerstedt G. 2007. Magn. Reson. Chem. 45:37-45.